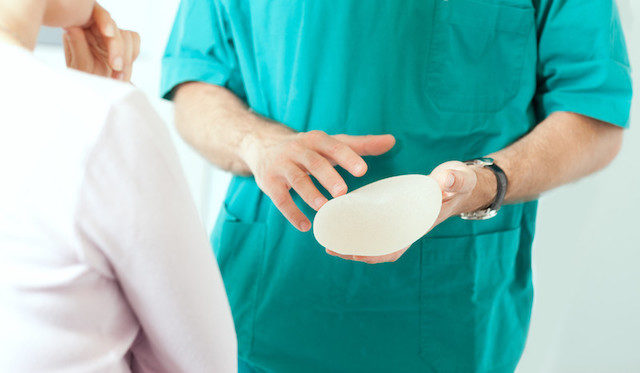
Allergan, a company that produces breast implants, just issued a full-scale recall of its Biocell textured implants that have been linked to cancer. A statement from Allergan said that it was recently made aware of a high incidence of a blood cancer called anaplastic large cell lymphoma (ALCL) in people who had used their implants.
Allergan, a company that produces breast implants, just issued a full-scale recall of its Biocell textured implants that have been linked to cancer. A statement from Allergan said that it was recently made aware of a high incidence of a blood cancer called anaplastic large cell lymphoma (ALCL) in people who had used their implants.
Who’s at risk?
These textured implants are far less common in the United States than they are in other countries. In fact, they account for only 5% of all implants sold in the US. However, because so many women have implants in general, that 5% encompasses more women than you would think. Dr. Binita Ashar from the Food & Drug Administration says that hundreds of thousands of women have these textured implants.
The specific implants that Allergan recalled are the Natrelle Saline-Filled breast implants, Natrelle Silicone-Filled breast implants, Natrelle Inspira Silicone-Filled breast implants, and Natrelle 410 Highly Cohesive Anatomically Shaped Silicone-Filled breast implants.
FDA Recommendations and Response
The FDA first learned of the connection between breast implants and cancer in 2011. The administration says the implants should only be removed if ALCL has been diagnosed. Women who have the implants and have not been diagnosed should simply monitor those areas. They can then talk to their doctor and potential symptoms or changes they notice. The main symptoms are swelling or pain around the implant.
Nearly 600 cases of ALCL related to breast implants have been reported, and it has claimed the lives of 33 people. Dr. Amy Abernethy of the FDA said once the association between the product and the disease was clear, the administration decided a recall was warranted and informed Allergan of their decision. She also said that they will continue to monitor the effect of all types of breast implants and will not hesitate to take further action if they can help and protect other patients.
The FDA’s Medical Devices Advisory Committee recently got together to discuss how they would monitor this problem. Dr. Jeff Shuren said they will continually renew any new information that becomes available regarding the connection and, if warranted, take further action. He also said they are still evaluating if ALCL is limited to certain kinds of textured implants or if it can be connected to all textured implants. The administration is also continuing to advise women and health care providers of the risks associated with implants.
The FDA also may choose to require that the labeling on breast implants include a portion describing the health risks. They consider the sharing of safety information to be vital in combating this problem.
These are not lifelong devices.
 According to the American Society of Plastic Surgeons, breast implants were the most performed cosmetic surgery last year. The surgery causes no problems for most women, but one in every five eventually have to have their implants removed. Dr. Tommaso Addona says that the implants are not meant to be lifetime devices and should generally be used for a period of 7-10 years.
According to the American Society of Plastic Surgeons, breast implants were the most performed cosmetic surgery last year. The surgery causes no problems for most women, but one in every five eventually have to have their implants removed. Dr. Tommaso Addona says that the implants are not meant to be lifetime devices and should generally be used for a period of 7-10 years.
Addona said he always discusses risks and complications with his patients prior to performing any implantation surgery. There can be scarring in the surgery area or soreness, and he also makes sure to disclose the association textured implants have with ALCL.