The U.S. Food and Drug Administration (FDA) has now approved the checkpoint inhibitor drug Keytruda to treat stage-3 melanoma after patients have undergone surgery. A phase 3 clinical trial revealed that patients who received Keytruda had a 43% decreased risk of disease recurrence or death.
What is Keytruda and how does it work?
 Keytruda is a type of immunotherapy treatment called a checkpoint inhibitor drug, and it works to stop something called a checkpoint protein from doing its job. Checkpoint proteins are important components of the immune system, as they prevent the body from attacking its own normal tissues. As many scientists describe it, they act as the “brakes” when the immune system tries to target cells that don’t need targeting. Unfortunately, cancerous cells have used checkpoint proteins to evade the immune system’s detection; even though cancerous cells are “foreign” to the immune system, checkpoint proteins prevents it from attacking, and cancer thrives.
Keytruda is a type of immunotherapy treatment called a checkpoint inhibitor drug, and it works to stop something called a checkpoint protein from doing its job. Checkpoint proteins are important components of the immune system, as they prevent the body from attacking its own normal tissues. As many scientists describe it, they act as the “brakes” when the immune system tries to target cells that don’t need targeting. Unfortunately, cancerous cells have used checkpoint proteins to evade the immune system’s detection; even though cancerous cells are “foreign” to the immune system, checkpoint proteins prevents it from attacking, and cancer thrives.
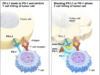
Researchers have discovered that checkpoint inhibitor drugs can prevent these “brakes” from working so that the immune system can properly attack cancerous cells. These drugs have been successful at treating many cancers, especially skin and lung. Keytruda was developed by Merck, and it works by binding to a protein called PD-1 on immune T cells. It then blocks interaction with its ligand PD-L1, which normally allows cancerous cells to evade immune detection.
What did the latest study on Keytruda show?
 The study, called KEYNOTE-054, involved a group of patients with stag-3 melanoma. Stage 3 means that the cancer has spread to the lymph nodes but not to other organs. The study’s participants all had previous surgery to remove the tumor. This particular group was also at a high risk of the melanoma coming back. A random selection of the total 1,019 patients were chosen to receive Keytruda, and the rest were given a placebo drug. Participants received the drug or placebo every three weeks for a year until disease recurrence or adverse side effects occurred.
The study, called KEYNOTE-054, involved a group of patients with stag-3 melanoma. Stage 3 means that the cancer has spread to the lymph nodes but not to other organs. The study’s participants all had previous surgery to remove the tumor. This particular group was also at a high risk of the melanoma coming back. A random selection of the total 1,019 patients were chosen to receive Keytruda, and the rest were given a placebo drug. Participants received the drug or placebo every three weeks for a year until disease recurrence or adverse side effects occurred.
The study’s end results revealed that 75.4% of patients who received Keytruda were alive and cancer-free for at least one year, compared to 61% of patients who received the placebo drug. Keytruda thus reduced the risk of disease recurrence or death by 43%.
 While the study was designed to test the efficacy of Keytruda in preventing the spread or recurrence of melanoma, it also aimed to determine the drug’s safety. According to the report, 14.7% of patients taking Keytruda did experience negative side effects, including one patient who died of chronic, progressive muscle inflammation, a known side effect of the drug.
While the study was designed to test the efficacy of Keytruda in preventing the spread or recurrence of melanoma, it also aimed to determine the drug’s safety. According to the report, 14.7% of patients taking Keytruda did experience negative side effects, including one patient who died of chronic, progressive muscle inflammation, a known side effect of the drug.
Overall, the study’s results were very promising, allowing Keytruda to become FDA-approved for treating stage-3 melanoma after surgery. Researchers and doctors are enthusiastic about the potential Keytruda has in helping to prolong the life of patients with melanoma.
Is Keytruda right for you?
Consult with your oncology team to determine whether Keytruda could be effective for your particular cancer.