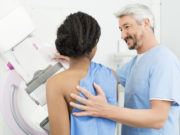
The U.S. Food and Drug Administration (FDA) announced yesterday that it has approved the first immunotherapy drug to treat breast cancer. The approval arrived after a study from the New England Journal of Medicine reported the drug stopped cancer progression in several patients.
The U.S. Food and Drug Administration (FDA) announced yesterday that it has approved the first immunotherapy drug to treat breast cancer. The approval arrived after a study from the New England Journal of Medicine reported the drug stopped cancer progression in several patients.
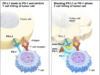
The treatment involves a combination of immunotherapy and chemotherapy with drugs called Tecentriq and Abraxane. Tecentriq is the immunotherapy portion of the treatment, and it works by allowing the immune system to detect and attack cancerous cells. The chemotherapy, Abraxane, then helps to destroy the cancerous cells after the immune system has been boosted.
Immunotherapy has become a standard way to treat several other cancers, but this is the first drug approved to treat breast cancer. Unfortunately, cancer has found ways to avoid detection from the immune system so it can continue to grow unharmed. Immunotherapy drugs thus attach to cancerous cells, getting rid of a protein called PD-L1, which is what helps cancer cells to “hide” from attack.
The treatment protocol is approved for patients with triple-negative breast cancer, local or metastatic, that is unable to be removed in surgery. In order for the immunotherapy to work, that patient’s cancer cells must also include the protein PD-L1.
The clinical study performed back in October showed that the treatment regimen lengthened “progression-free survival” time, which refers to the amount of time during which a patient does not experience disease growth. In patients who had PD-L1 cancerous cells, and received the combination treatment, the median progression-free survival was 7.4 months in comparison to 4.8 months for patients receiving chemotherapy with a placebo drug.
According to Dr. Peter Schmid of Queen Mary University of London, the study’s lead author, “This is the first time immunotherapy has worked in such a difficult to treat cancer, and is a huge step forward for these breast cancer patients.”
 According to the National Breast Cancer Foundation, triple-negative breast cancer makes up about 15% of all breast cancers. Studies have also shown that about one fifth of those cancers contain the PD-L1 protein.When breast cancer cells do not contain estrogen, progesterone, or human epidermal growth factor 2 (HER2,) they are considered triple-negative. This also means that the cancer doesn’t respond to any available hormonal therapies. It is a particularly aggressive cancer because it develops a quick resistance to chemotherapy drugs, allowing for the cancer to spread to other organs.
According to the National Breast Cancer Foundation, triple-negative breast cancer makes up about 15% of all breast cancers. Studies have also shown that about one fifth of those cancers contain the PD-L1 protein.When breast cancer cells do not contain estrogen, progesterone, or human epidermal growth factor 2 (HER2,) they are considered triple-negative. This also means that the cancer doesn’t respond to any available hormonal therapies. It is a particularly aggressive cancer because it develops a quick resistance to chemotherapy drugs, allowing for the cancer to spread to other organs.
The treatment protocol received the FDA’s accelerated approval, which means that its approval process was sped up in order to benefit patients immediately. This also means that the drug was based on the study’s results showing “progression-free survival,” and researchers hope that future data will show the treatment extending lives. Trials will continue to confirm the drug’s efficacy which could then allow it to receive a traditional FDA approval. If the drug does not continue to show success, the FDA could also pull it.
But right now, researchers, doctors, and breast cancer patients are thrilled about the opportunity to use the immunotherapy regimen. Dr. Larry Norton, director of the Evelyn H. Lauder Breast Center at Memorial Sloan Kettering Cancer Center told CNN that “this is just the beginning of using immunotherapy for breast cancer.”