The federal “Right to Try” law makes it possible for terminally ill patients to access investigational treatments that are not yet approved by the Food and Drug Administration (FDA). Although the law has been previously passed in 41 states, President Trump made it legal throughout the entire country when he signed the bill last May.
 Trump acknowledged this achievement in his State of the Union address early this week, which left many people questioning whether the law is really working and why we even needed it in the first place. Well, we know from public reports that the law has been helping at least two patients access treatments, and according to the official Right to Try website, several more have also benefited.
Trump acknowledged this achievement in his State of the Union address early this week, which left many people questioning whether the law is really working and why we even needed it in the first place. Well, we know from public reports that the law has been helping at least two patients access treatments, and according to the official Right to Try website, several more have also benefited.
The law has sparked much political and philosophical debate. But when it’s broken down, it really does seem to be a bipartisan issue. No matter where you stand politically, if you’re diagnosed with a terminal illness and there’s a drug that may help you, you’ll likely be glad that Right to Try exists. Here are 5 critical reasons why.
Most terminally-ill patients aren’t eligible for clinical trials.
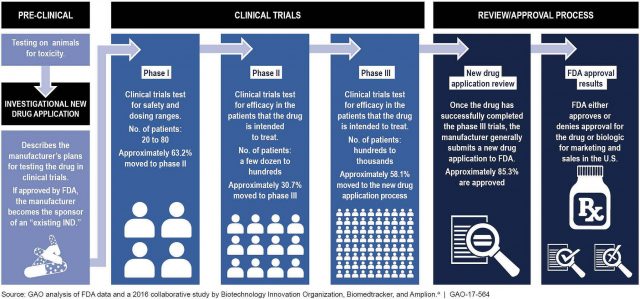
 There are a few conditions that need to be met in order for someone to access treatments under Right to Try. The drug in question must have already passed an FDA-approved Phase 1 clinical trial, and it must also be in a currently-active clinical trial on a path to approval. The patient seeking treatment cannot be eligible for a clinical trial involving the treatment they want to use.
There are a few conditions that need to be met in order for someone to access treatments under Right to Try. The drug in question must have already passed an FDA-approved Phase 1 clinical trial, and it must also be in a currently-active clinical trial on a path to approval. The patient seeking treatment cannot be eligible for a clinical trial involving the treatment they want to use.
Although clinical trials with the drugs in question are likely available, terminally ill patients usually don’t qualify for them. This is because their disease is typically too advanced or they are not physically well enough to participate. Right to Try thus offers them an alternative way to still access the potentially-helpful treatment.
When clinical trials end, many patients are still left without options.
The lucky few patients who are eligible for clinical trials sometimes see great results. But once the trial is over, they don’t have access to the treatment that may have been working. Even if the treatment was a success and will go on to receive FDA approval, that process takes a long time. Those with life-threatening diseases usually don’t have the time to wait around for that approval. Right to Try offers them another opportunity to access the drug that could extend their life.
The FDA’s “Compassionate Use” is limited.
Although the FDA does have “Compassionate Use,” which allows terminally-ill patients to fill out applications to access investigational drugs, the program is not benefiting many patients. This is because the application process isn’t very straightforward. Not only is it lengthy and difficult, it also costs a lot of money. This means that not many people can actually complete it. Again, when patients are terminal, they don’t have the time to wait around. Right to Try is a way of getting around some of this “red tape.”
The drug approval process takes too long to benefit terminal patients.
 Drugs can take as long as ten years to be officially approved by the FDA. Many terminally-ill patients will die in the years it takes for treatments to become available to them. Right to Try gives them access to these investigational treatments when they need them the most: right now.
Drugs can take as long as ten years to be officially approved by the FDA. Many terminally-ill patients will die in the years it takes for treatments to become available to them. Right to Try gives them access to these investigational treatments when they need them the most: right now.
Patients should have the right to try and save their own lives.
As ALS-patient and avid Right to Try supporter asked Congress during one of his many testimonies, “If I’m not going to make it anyway, why shouldn’t I get to try?”
The U.S. Constitution is based on the very “American” principle of freedom. Right to Try advocates that every terminally-ill patient should get to take advantage of that freedom in the form of choosing to try and save their own lives. Crucial decisions like these should not be left up to the federal government. When they utilize right to try, patients access the freedom they deserve to try any treatment that exists.