The U.S. Food and Drug Administration (FDA), will be reviewing Genetech’s Tecentriq (atezolizumab) matched with the chemotherapy drug Abraxaine (nab-paclitaxel) and carboplatin for metastatic non-squamous non-small cell lung cancer treatment. The drugs target demographic will be NSCLC patients with no mutations in the estimated glomerular filtration rate, known as EGFR and anaplastic lymphoma kinase, called ALK genes.
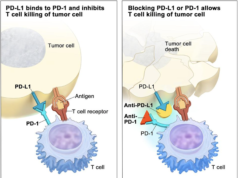
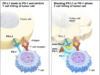
Cancerous cells are shown to use their own programmed death-ligand 1, or PD-L1 proteins and fuse to the programmed cell death, or PD-1 receptors on immune cells. This is so they can specifically avoid the systems attack. Tecentriq(atezolizumab) is a checkpoint inhibitor drug that is aimed directly to target the PD-L1 protein. The inhibitor therapy deflects the bond, and allows the immune system to attack the tumors or malignant cells.
The clinical trial, included over 700 untreated metastatic NSCLC patients. Of the trial pool nearly 500 patients were selected at random to receive the Genetech’s test trial treatment. The treatment consisted of the Tecentriq (atezolizumab) triple compound, with the remaining patients treated solely with chemotherapy.
Seeking to prove if Tecentriq was able to increase the overall survivor rate and slow the progression of the NSCLC disease for an extended amount of time, rather than the isolated treatment use of chemotherapy-patients were supplied with a maximum of 6 cycles of chemotherapy, while receiving Tecentriq. The controlled patient pool was treated with continuous doses of Alimta (pemetrexed). Within that same group some participants were given the opportunity to change their treatment to Tecentriq, until an increased progression of the NSCLC disease was evident.
As a result, the trial outcomes were successful. Researchers observed a 13.9 months -18.6 months prolonged life survival. Additionally, the clinical trial boosted a 36% reduction of death or progression of the NSCLC disease. The data also showed, that the Tecentriq therapy combination is as safe to the human body as its individual elements. Approximately 73% of the patients that received the tested therapy combination, displayed body or life damaging adverse effects, as compared to the 60.3% of patients receiving only chemotherapy.
Treatment combinations previously approved by the U.S Food and Drug Administration (FDA), for patients with non-squamous NSCLC patients without EGFR or ALK mutation tumors includes Tecentriq combined with Avastin (bevacizumad) and the chemotherapy Taxol (paclitaxel) and carboplatin. Genetech hopes to receive the FDA review of their new treatment drug by the expected completion date, September 2, 2019.