Early results from a phase one clinical trial showed that a new colorectal cancer vaccine could be an effective way to treat the disease. The study, published in the Journal for Immunotherapy of Cancer, revealed that the vaccine safely activated patients’ immune system, which will allow the treatment to move on to a larger study.
Early results from a phase one clinical trial showed that a new colorectal cancer vaccine could be an effective way to treat the disease. The study, published in the Journal for Immunotherapy of Cancer, revealed that the vaccine safely activated patients’ immune system, which will allow the treatment to move on to a larger study.
Colorectal cancer is a difficult cancer to catch early since most f the symptoms only show up when the cancer is advanced. The cancer is also known to recur often, even after patients undergo surgery. Because the disease is the second leading cause of cancer-related deaths in the world, this new immunotherapy treatment could save many lives.

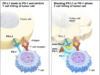
The treatment uses a vaccine that helps to trigger a patient’s immune system so that it can more effectively attack cancerous cells. The immune system is built to fight off foreign invaders, but most of the time, it has a hard time recognizing cancerous cells as “foreign.” Immunotherapy treatments are focused on getting the immune system to be aware that cancerous cells exist so that it can do its job in destroying them.
The study was based on the discovery that nearly all colorectal cancers express a molecule called GUCY2C. That molecule is found in intestinal epithelial cells as well, but when the vaccine targets it, it theoretically should only send immune cells to tumors rather than including healthy tissue as well.
The phase one of the trial intended mostly to determine the safety of the treatment. Ten patients who either had stage I or II colon cancer were given a single dose of the vaccine and followed for six months. None of these patients had serious adverse effects, and blood samples showed that the vaccine was successful at increasing the kind of immune activity that helps create anti-tumor immune cells.
According to Karen Knudsen of the Sidney Kimmel Cancer center, “this pivotal study provides some of the first evidence that it may be possible to safely direct a patient’s own immune system to seek and destroy this cancer type.”
 The treatment is only in its early phases of study, and the next step is to enter larger Phase II trials which will include more patients and a focus on evaluating the efficacy of the vaccine. The formula has already been modified to hopefully ensure that it will be even more effective in the next round of patients. Researchers also believe that the vaccine could be successful for more than just colorectal cancer, as studies have shown that the GUCY2C molecule is also expressed by gastric, esophageal, and pancreatic cancers.
The treatment is only in its early phases of study, and the next step is to enter larger Phase II trials which will include more patients and a focus on evaluating the efficacy of the vaccine. The formula has already been modified to hopefully ensure that it will be even more effective in the next round of patients. Researchers also believe that the vaccine could be successful for more than just colorectal cancer, as studies have shown that the GUCY2C molecule is also expressed by gastric, esophageal, and pancreatic cancers.